WHY THIS MATTERS IN BRIEF
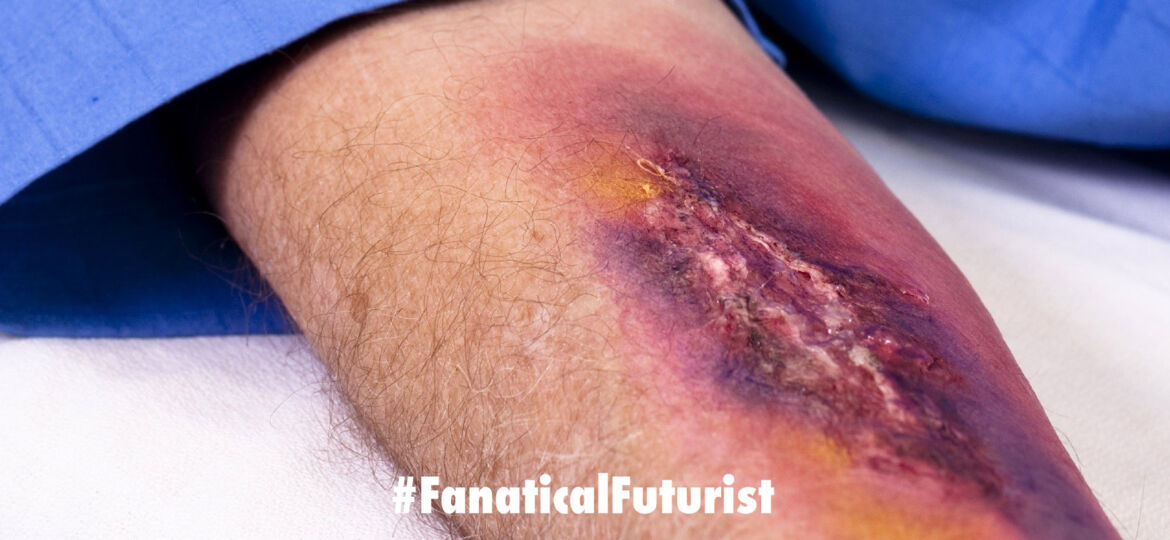
Treating third degree burns today often involves skin grafts and more pain for the patient, spray on skin will put an end to that misery.
Over the past couple of years we’ve gotten increasingly good at 3D printing human skin and making artificial skin that, for example, can be used to create next generation prosthetics and robots that can “feel,” and now there’s even more good news, especially for the millions of people who suffer severe burns and abrasions every year. ReCell, a skin cell solution, which is one of several revolutionary “spray on skin” regenerative medicine products, is the first treatment of its kind approved by the US Food and Drug Administration for growing back skin.
In the US alone, roughly half a million people have to seek medical treatment for their burns each year. The most traditional form of treatment for bad burns comes in the form of skin grafts. However, those require more pain on the part of the patient, as doctors have to cut away and remove healthy skin elsewhere on the body to cover the burns.
ReCell is the creation of AVITA Medical, a global regenerative medicine company. According to CEO Michael Perry, the company wanted to give burn patients more treatment options – especially ones that were less painful.
“Today’s approval of the ReCell System marks an important milestone for us and provides a new way to treat burns for the thousands of patients with significant unmet medical needs, said Perry in a statement.
“We are grateful to those patients who participated in clinical trials of the RECELL System and to the clinical trial investigator teams whose dedication and scientific rigor made this approval possible. We also greatly appreciate our collaboration with BARDA and the support that they have provided to us throughout the development of the RECELL System.”
ReCell is definitely not the only solution that’s been in the works over the past years though, it’s just the first one to get the magical FDA stamp of approval which means they can now sell it to healthcare providers throughout the US.
In 2009, MIT researchers made headlines when they proposed a similar spray-on skin solution, and elsewhere RenovaCare also created the SkinGun which I talked about a little while ago.
However, Avita’s technology beat its competitors to FDA approval, and possibly to the market, first.
ReCell reduces how much skin has to be removed over the burned surface before treatment, the company explained. It uses enzymes to break down those layers of skin from a piece of tissue. It then mixes those tissue cells into a liquid that’s applied to the skin using a simple, low-tech spray.
In most burn scenarios, skin grafts can require more skin than a patient anticipates. There’s also nerve damage along with damaging skin and muscle tissues.
Perry told media outlets that ReCell reduces the amount of healthy skin that gets damaged during a skin graft by 97 percent for a second degree burn, and even more for a third degree burn.
For doctors, ReCell could give them a new way to treat patients faster, easier, and safer than ever before. It takes just 30 minutes to process a patient’s skin, and because it’s the patient’s own skin from the area, there’s next to no possibility of rejection.
“Today’s approval of the RECELL System is a significant advancement in how we treat patients with burns,” said James H Holmes IV, MD, FACS, Wake Forest Baptist Medical Center, Winston-Salem, North Carolina. “Dramatically reducing the amount of donor skin needed to treat second and third degree burns has important implications for pain, scarring and costs of care, while still providing comparable healing to the current standard of care. Additionally, the potential reduction in mortality is extremely promising.”
Currently, AVITA hasn’t listed a price for ReCell. However, Perry told NBC News it could be between $5,000 to $10,000 per unit. That cost would cover roughly 10 percent of a patient’s body. Deeper burns or burns covering a larger surface area would naturally require more units.
Source: AVITA