WHY THIS MATTERS IN BRIEF
In the future not all organisms will be children of Mother Nature …
 Love the Exponential Future? Join our XPotential Community, future proof yourself with courses from XPotential University, connect, watch a keynote, or browse my blog.
Love the Exponential Future? Join our XPotential Community, future proof yourself with courses from XPotential University, connect, watch a keynote, or browse my blog.
Synthetic cells and basic synthetic organisms that have no equal or equivalent in nature aren’t anything new – although they’ve only really “existed” for a couple of years now and the field is still in its earliest stages. But now scientists have crafted a single-celled synthetic organism that divides and multiplies just like the real thing, and the development could someday help researchers to build miniscule “living” computers and tiny drug-producing factories, all out of synthesized cells – any one of which would be a world changing revolution in itself. But, of course, that future likely won’t be realized for many years to come.
“There’s just so many ways in which this coming century of [synthetic] biology could potentially change our daily lives for the better,” said senior author Elizabeth Strychalski, leader of the Cellular Engineering Group at the National Institute of Standards and Technology (NIST) in the US who led the team. For example, Strychalski and her colleagues plan to engineer living sensors, like the ones I’ve talked about at length recently, that can take measurements from their surrounding environments, monitoring the acidity, temperature and oxygen levels nearby. These “sensor cells” could also be manufactured to produce specific products — namely medicines — and could potentially be placed inside the human body itself.
“One vision is that when the cell senses a disease state, then it can make that therapeutic, and when a disease state is longer there, they could stop making that therapeutic,” Strychalski said – something that, again, would be revolutionary and forever change the healthcare industry. Other cells could be cultured in the lab and used to efficiently produce food and fuel products, while still others could be made to perform computational functions at a molecular scale, she added.
But again, these are all visions for the future. To get there, scientists need to unpack the mysteries of the cell at a fundamental level before they can manipulate it in their synthetic organisms.
In the new study, Strychalski and her colleagues took a step toward that goal and published their results March 29 in the journal Cell. They began with an existing synthetic cell called JCVI-syn3.0, which was created in 2016 and contains only 473 genes, Scientific American reported. For comparison, the bacterium Escherichia coli has about 4,000 genes, according to a statement.
This bare-bones cell was crafted from the bacterium Mycoplasma genitalium, a sexually transmitted microbe, which scientists stripped of its natural DNA and replaced with their own synthetic DNA. In creating JCVI-syn3.0, the scientists wanted to learn which genes are absolutely essential for a cell to survive and function normally, and which are superfluous.
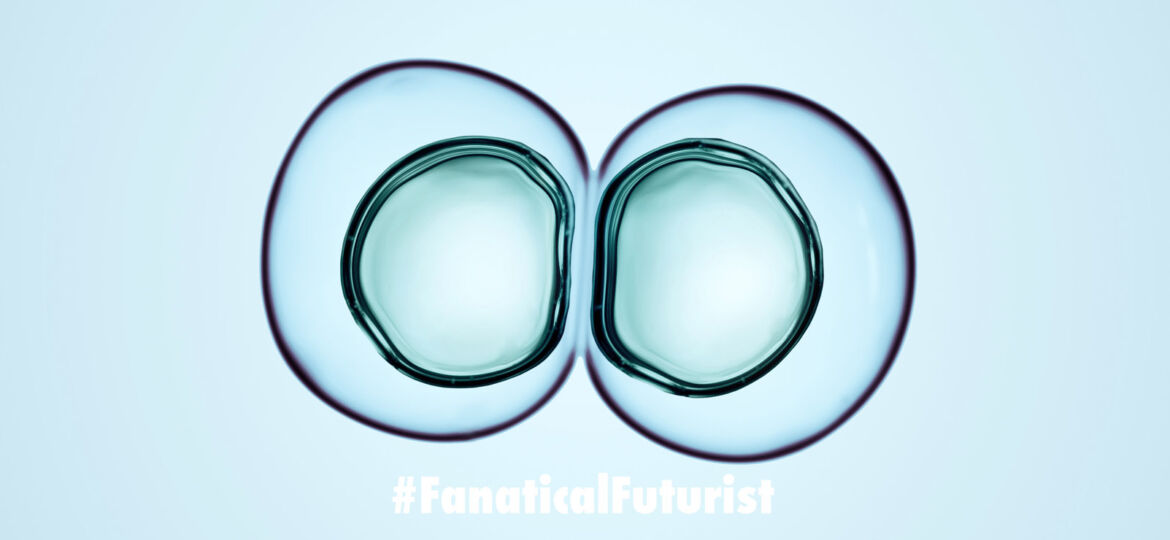
But while JCVI-syn3.0 could build proteins and replicate its DNA without issue, the minimalist cell could not divide into uniform spheres. Instead, it split haphazardly, producing daughter cells of many different shapes and sizes. Strychalski and her team set out to fix this problem by adding back genes to the stripped-down cell. After years of work, the scientists produced JCVI-syn3A, which contains a total of 492 genes. Seven of these genes are critical for normal cell division, they discovered.
“A number of the genes in the minimal cell did not have a known function,” said co-first author James Pelletier, who at the time of the work was a graduate student at MIT, adding, “It turned out that some of the genes that the cell needs to divide previously did not have a known function.” Reintroducing these genes allowed the minimal cell to split into perfectly uniform orbs.
Some of these important genes likely interact with the cell membrane, based on their genetic sequences, Pelletier said. This could mean that they alter the physical properties of the membrane, making it malleable enough to divide properly, or that they generate forces within the membrane that encourage the split, he said. But for now, the team doesn’t know what specific mechanisms the genes use to help cells split, he noted.
“Our study was not designed to figure out the mechanisms inside of the cell associated with each of these genes of unknown function,” Strychalski said. “That’s going to have to be a future study.”
While researchers continue to probe the mysteries of the minimal cell, other synthetic biologists are working with even more simplistic systems. Synthetic biology exists on a spectrum, from “a soup of inanimate chemicals to the full glory of a mammalian cell or a bacterial cell,” Strychalski said. The future of the field could lead us to innovative wonders like cell-sized biological computers, that are already emerging, but for now, the work is largely driven by a curiosity about how the basic building blocks of life come together, and what that can tell us about ourselves, she said.
“How do we understand the most basic unit of life, the cell? … There’s something very compelling about that,” Strychalski said. “Later on, we can imagine all the things we can do with … this minimal platform.”

















[…] In the future not all organisms will be children of Mother Nature … — Read on https://www.fanaticalfuturist.com/2021/04/scientists-built-a-perfectly-replicating-synthetic-cell/ […]