WHY THIS MATTERS IN BRIEF
Scientists are using the revolutionary gene editing technology CRISPR, dubbed the “Genesis Engine,” to treat, cure, and reverse a whole range of medical conditions that range from Cancer and HIV to blindness and inherited syndromes and the results so far are staggering.
Hailed as a breakthrough with the potential to correct genetic mutations and cure life threatening diseases, almost at will, the revolutionary gene editing technology CRISPR may also be able to reverse an inherited condition that causes blindness.
Scientists at Columbia University Medical Center and the University of Iowa have used CRISPR to repair a genetic mutation responsible for retinitis pigmentosa in induced pluripotent stem cells derived from a patient with the disease. Retinitis pigmentosa is a genetic eye condition that causes the retina to deteriorate, leading to a slow loss of vision and resulting in blindness in at least 1.5 million cases worldwide. There is no cure for retinitis pigmentosa, and the only treatment is vitamin A, which may help slow down the progression of vision loss in some people with the disease.
In the study, published in the journal Scientific Reports, researchers took a sample of skin from a patient with a mutation in the retinitis pigmentosa GTPase regulator (RPGR) gene, which causes an aggressive, X-linked variant of the disease. This common variant of the disease was pinpointed because it is caused by a single mistake in the RPGR gene, making it a good candidate for precision medicine.
“The X-linked form of retinitis pigmentosa is an ideal candidate for a precision medicine approach because a common mutation accounts for 90% cases,” said study author Dr. Stephen Tsang, an associate professor of pathology and cell biology at Columbia.
The scientists used an established method to generate pluripotent stem cells from the patient’s skin cells. These embryonic like stem cells have the potential to differentiate, or turn into, healthy retinal cells and be transplanted back into the same patient to treat vision loss.
But the stem cells derived from the patient with retinitis pigmentosa still harboured the disease causing mutation. So in an attempt to fix this abnormality, investigators used CRISPR to target, edit out and replace the mistake in RPGR. CRISPR is easily adaptable to diverse sequences of DNA, and the technology allows for faster and more accurate editing than previous methods.
The researchers then waited 10 days, collected the cells and performed DNA sequencing several times on the cells to see if the gene editing method worked.
In their published findings, the team reported a 13% success rate at converting the mutated gene variant into the normal one. That doesn’t seem like a high success rate, but the authors say it’s better that previous stem cell gene editing studies that have reported correction rates closer to 1%. But the authors are encouraged by the results, pointing out that the composition of RPGR – which contains many repeats and tight binding nucleotide pairs – makes it a difficult gene to edit.
“Our vision is to develop a personalised approach to treating eye disease,” Tsang said, “we still have some way to go, but we believe that the first therapeutic use of CRISPR will be to treat an eye disease. Here we have demonstrated that the initial steps are feasible.”
There have been a lot of firsts for CRISPR in the past few years, and the authors of this study say the eye is an ideal place to start for the first clinical use of CRISPR. Compared to other parts of the body, the eye is easy to access for surgery, readily accepts new tissue and can be noninvasively monitored.
The idea would be to transplant the corrected stem cells back into the patient. This procedure, though, is still being investigated as a safe and effective treatment for patients.
The first clinical trial using induced pluripotent stem cells to treat another eye condition, macular degeneration, began in September 2014. But the trial was halted last summer due to safety concerns after genetic mutations were discovered in the cells of a participant. If eventually successful, the trial would provide evidence of the clinical potential of these stem cells and pave the way for more human trials.
Other types of gene therapies for retinitis pigmentosa are currently undergoing clinical trials. These therapies differ from the CRISPR-based method in that they introduce stretches of DNA that supplement some of the activity of the defective gene rather than attempting to correct the original mutation itself.
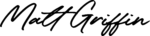
















[…] it’s a promising start and one day, just as we’ve recently seen how gene therapy can cure blindness, now we might also see it cure […]